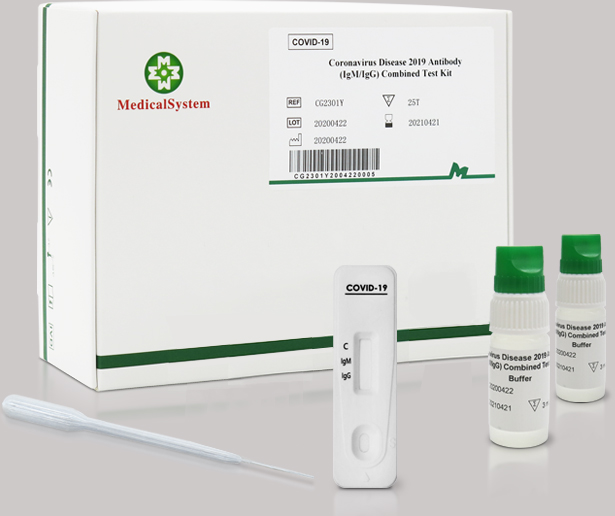
The COVID-19 IgG/IgM Rapid Test has emerged as a critical part of this arsenal, delivering swift diagnosis - with results in 15 minutes or less - in a variety of locations such as physicians' offices, urgent care clinics and other point-of-care locations.
Because of its portability and speed, rapid IVD testing has been crucial in battling COVID-19 across the globe.
FEATURES + BENEFITS
- Qualitative detection of IgG and IgM antibodies
that bind to COVID-19 causing virus
- Simple and easy to use
- Healthcare providers can administer tests at the
point-of care or laboratories
- Results delivered within minutes
DISCLAIMER
- COVID-19 IgG/IgM Rapid Test is a solid phase
immunochromatographic assay used in the
rapid, qualitative and differential detection of IgG
and IgM antibodies to the 2019 novel coronavirus
in human whole blood, serum or plasma.
- Results from antibody testing should not be
used as the sole basis to diagnose or exclude
SARS-CoV-2 infection or to inform infection status.
- Negative results do not rule out SARS-CoV-2
infection, particularly in those who have been in
contact with the virus.
- Follow-up testing with a molecular diagnostic
should be considered to rule out infection in these
individuals.
- Positive results may be due to past or present infection
with non-SARS-CoV-2 coronavirus strains, such as
coronavirus HKU1, NL63, OC43, or 229E.
- It is not known whether persons having antibodies to
SARS-CoV-2 are not infectious, can be re-infected or may
experience COVID-19 in the future.
- Not for use in blood screening.
- For use only by clinical laboratories and healthcare
workers at the point-of-care. All other use is prohibited.
- Laboratories within the United States and its territories
are required to report all positive results to the
appropriate public health authorities.
- Manufactured in China by MedicalSystem Biotechnology
Co., Ltd (SHE: CN: 300439), a publicly traded Chinese
company, distributed in the US by RapidIVD, a PMT
Medical company
- MedicalSystem Biotechnology has notified the FDA that
they have validated and will begin offering the COVID-19
IgG/IgM Rapid Test. The test is being made available
under compliance with Section IV.D. of the FDA's Policy
for Diagnostic Tests for Coronavirus Disease-2019 during
the Public Health Emergency. Updated FDA guidance,
issued on March 16, 2020, allows the distribution of this
product for diagnostic use by professionals within highly
complex settings and or pharmacies. The updated policy
can be viewed by clicking here.
- This test is FDA EUA approved, but has not been reviewed
by the FDA.